How to Find Molar Ratio of a Compound
The mole ratio is also called the mole-to-mole ratio. So you would use only the formula for NH3 to calculate the molar massTo calculate M multiply the molar mass of each element atomic weight in gmol by its subscript and add the results.
I have two volatile compounds such as ethyl acetate and ethyl propionate.

. Convert all masses into moles. To calculate the molar ratio from the mass of the reactants or products follow these simple steps. Click to see full answer.
Up to 24 cash back Also known as. A 12 --- one mole of CuCl 2 and two of water b 11 --- one mole of MgSO 4 and one of water c 118 --- one mole of Cr 2 SO 4 3 and eighteen of water. I am going to separate these compounds by simple and fractional distillation.
25 cm 3 25 1000 dm 3 25 10-3 dm 3. Molar mass is the mass in grams of one mole of a substance. Molar mass of CH 2 O 1 x 12 2 x 1 1 x 16 12 2 16.
Therefore the molar concentration of NaOH 87510 3 25010-3. Use the coefficients in front of compounds to get molar ratios. Write the chemical formula of.
The molar ratio from the balanced equation must be considered to tell us how many moles of aluminium will. Now we have the equation First convert the volume of aqueous NaOH into dm-3. The molar mass was given as 60 gmol.
For every 1 mole of O2 used 2 moles of H2O are formed. Method 1Method 1 of 2Calculating the Molar Mass of an Element. For example - analysis of a sample of water would reveal that the number of moles of hydrogen atoms present in the sample is twice the number of moles of oxygen atoms in the sample.
Dividing each number by the number of moles of the element present in the smaller amount gives. Calculate the molar mass of each substance by evaluating their molecular weight. Using the atomic mass of an element and multiplying it by the conversion factor grams per mole gmol you can calculate the molar mass of that element.
The coefficient number in front is the number of moles of that compound. First you should know the molecular weight for each compound then use this weight as one mole weight for example if you want to make a. Calculate the molar ratios.
Identify the Mole Ratio of the given compound. What is the molar ratio between the anhydrous compound and water for the following hydrates. For every 2 moles of H2 used 2 moles of H2O are formed.
You can use the mole ratio of elements in a compound to determine the empirical formula of a compound. Then find the ratio of the masses of copper in the two compounds by dividing the larger value by the smaller value. Find the number of atoms of each element from the Mole Ratio.
1 mol Zn. Round off the mole ratio to the nearest whole number if less than 019 or greater than 081 fractions. 1568 10 3 1254 10 3 1250.
Since the moles of O is still not a whole number both moles can be multiplied by 2 while rounding to a whole number. A 25 to 1 is really a 5 to 2 ratio. Otherwise multiply the mole ratio by a number which gives two whole numbers for the ratio within 01 range of the whole number.
F is the number of reactive groups per mole of compound. The mole ratio between H2 and H2O is 11. Multiply each of the moles by the smallest whole number that will convert each into a whole number.
FeO 2 115 23. 1254 10 3 1254 10 3 1000 C. Then we should find out how much CH 2 O units are present there.
To find the molecular formula the integer need to multiply the. Find the molecular formula. 120g Al 1 mol Al 2698g Al 0044 48 mol Al 240g I₂ 1 mol I2 2538g I₂ 0.
1 mol ZnCl2. For each compound find the grams of copper that combine with 100 g of chlorine by dividing the mass of copper by the mass of chlorine. The polyol is a trihydroxy compound and therefore has an f of 3 while the diidsocyanate and chain extender have fs.
Zn s 2HCl aq ZnCl2aq H2g The molar ratio is. The molar mass of a compound with the empirical formula eqCH_2O eq is 8206 gmol. Then do you use coefficients when calculating molar mass.
A CuCl 2 2H 2 O b MgSO 4 H 2 O c Cr 2 SO 4 3 18H 2 O. Number of moles massformula mass. 2 mol HCl.
MolesC 1883 10 2gC 1molC 12011gC 1568 10 3molC. For that first calculate the molar mass of empirical formula and then divide the given molar mass value from the calculated value. MolesH 1264 10 3gH 1molH 10079gH 1254 10 3molH.
This lets us conclude that the empirical formula of water is H_2O which is also. Check to make sure you use the appropriate number of significant figures for atomic masses and report mass using the correct number of figures. The keys to getting the correct answer are.
The amount of NaOH present in moles is 875 10-3 mol. Apply the law of multiple proportions to the two compounds. The non-whole number empirical formula of the compound is Fe 1O15.
Divide the mass of the first substance by its molar mass to obtain the number of moles used or produced in the reaction. For the reaction2 H2g O2g 2 H2Og The mole ratio between O2 and H2O is 12. The mole ratio NaOH.
Make sure the chemical equation is balanced. How do I calculate mole ratios of the two compounds and determine the boiling point temperatures of the first drop based on the liquid and vapor phase diagram. Also determine the water in the hydrate.

Question Video Deducing The Molar Ratio Of Reactants From A Balanced Reaction Equation Nagwa

Question Video Determining The Molar Ratio Of Two Reactants Given The Mass And Molar Mass Nagwa

Stoichiometry Mole To Mole Conversions Molar Ratio Practice Problems Youtube
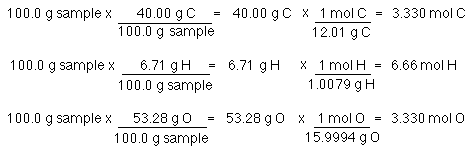

No comments for "How to Find Molar Ratio of a Compound"
Post a Comment